A new type of electrode that could lead to the development of more efficient and lighter supercapacitors has been unveiled by researchers in India. The electrode has a new hybrid structure that is made from iron and nickel nanowires, and could be used to boost the capacitance, current density and charging/discharging rates of big capacitors used to store large amounts of electrical energy. The electrodes are inexpensive and environmentally friendly to produce, say the researchers, and could someday be used to make supercapacitors to power a range of devices, from mobile phones to electric cars.
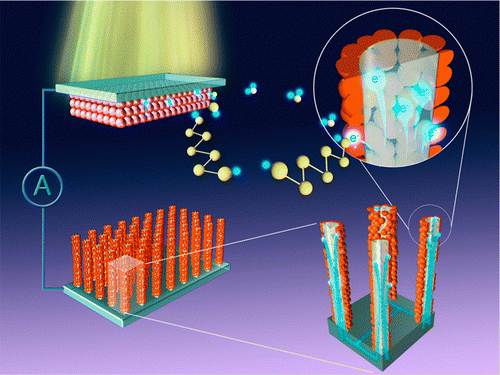 Supercapacitors store energy by separating positive and negative charge through electrochemical reactions that involve the exchange of electrons and ions at the interfaces between two electrodes and an electrolyte. These devices combine the large-scale energy-storage properties of batteries with the rapid charging times and long lifespans of conventional capacitors. In principle, supercapacitors could be used to create electric cars that could be fully charged in minutes, and mobile phones that would charge in seconds. Today, however, a supercapacitor is much larger and heavier than a conventional battery that holds the same amount of energy.
Supercapacitors store energy by separating positive and negative charge through electrochemical reactions that involve the exchange of electrons and ions at the interfaces between two electrodes and an electrolyte. These devices combine the large-scale energy-storage properties of batteries with the rapid charging times and long lifespans of conventional capacitors. In principle, supercapacitors could be used to create electric cars that could be fully charged in minutes, and mobile phones that would charge in seconds. Today, however, a supercapacitor is much larger and heavier than a conventional battery that holds the same amount of energy.
Created by Ashutosh Singh and colleagues at the S N Bose National Centre for Basic Sciences in Kolkata, the new electrode has a two-part nanostructure comprising a conductive iron–nickel core and a hybrid iron-oxide–nickel-oxide outer shell. The electrodes are made in two stages. First, arrays of iron–nickel nanowires are created through electro-deposition into a porous, anodized alumina-oxide template. After the template is dissolved away, the second step of the process sees the wires temporarily exposed to oxygen at a temperature of 450°. This forms a porous iron-oxide–nickel-oxide hybrid shell around the iron–nickel core.
With the initial tests of their demonstration electrode complete, the researchers are now moving to develop a functioning supercapacitor based around the hybrid-electrode design. Then they will test the functional performance and temperature stability of the device. A complete supercapacitor is expected to be developed in about 8–10 months, Singh told physicsworld.com. The researchers say that they will also be exploring avenues of commercial production.